Difosforus Pentaoksida
1144590 what i knowchoose the letter of the best answer.
Difosforus pentaoksida. Now diphosphorus pentoxide reacts violently with water to form phosphoric acid h3po4. Write the chosen letter on a separate sheet ofpaper1. M p 30973762 gmol.
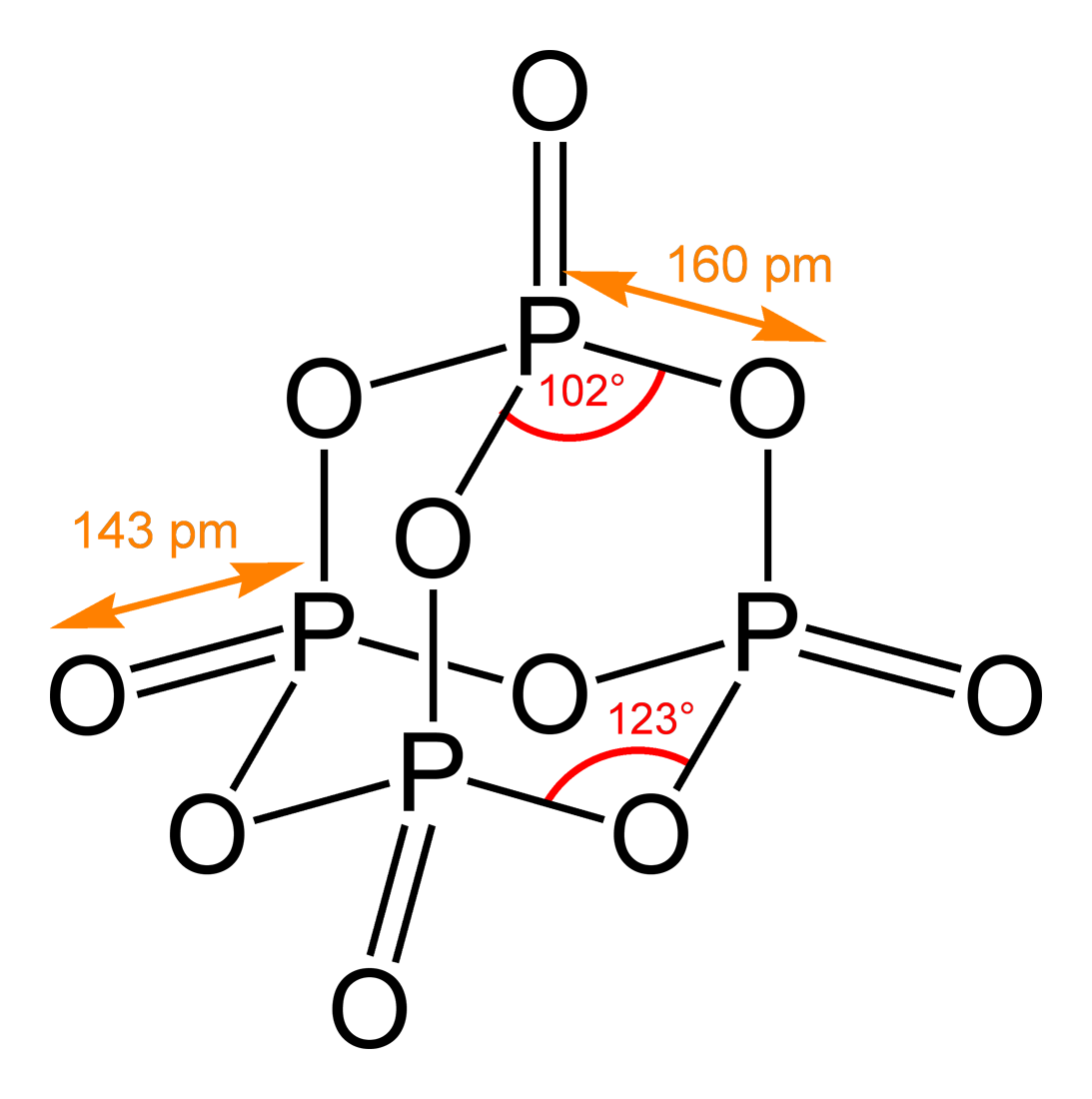
Diphosphorus pentoxide is a covalent compound that has an empirical formula of p 2 o 5 and a chemical formula of p4o10. Thats the formula for diphosphorus pentoxide a chemical that causes skin burns eye injury and pulmonary edema and which may not be the best name for a restaurant. Phosphorus pentoxide is a deliquescent compound prepared by reacting phosphorus with air.
They can be reduced to primary amines through the action of lithium aluminum hydride or hydrolyzed to carboxylic acids in the presence of either an acid or a base. This implies that p2o5 is actually the compounds empirical formula and that you should use p4o10 as its molecular formula. The interesting thing about diphosphorus pentoxide p2o5 is that it usually exists as a dimer.
The molar masses of phosphorus p and oxygen o are. What is the the formula of diphosphorus pentoxide is. P 4 o 10 6 h 2 o 4 h 3 po 4 177 kj.
Process for producing halogenated heteroaryl compounds. Application dehydrating agent used for halogenation with tetrabutylammonium halides. Phosphorus pentoxide is a potent dehydrating agent as indicated by the exothermic nature of its hydrolysis.
Phosphorus pentoxide phosphoric anhydride phosphorusv oxide. Formed by heating amides with phosphorous pentoxide. If phosphorus pentoxide contacts the eyes severe irritation and burns blepharospasm spasmodic winking lacrimation tearing and photophobia heightened sensitivity to light may occur.
Eye contact may lead to a total destruction of the eyes.

5 Persamaan Reaksi Ppt Reaksi Kimia Meliputi Perubahan Satu Atau Lebih Zat Menjadi Satu Atau Lebih Zat Lain Atau Dapat Juga Dikatakan Reaksi Kimia Course Hero
www.coursehero.com

5 Persamaan Reaksi Ppt Reaksi Kimia Meliputi Perubahan Satu Atau Lebih Zat Menjadi Satu Atau Lebih Zat Lain Atau Dapat Juga Dikatakan Reaksi Kimia Course Hero
www.coursehero.com

Tata Nama Senyawa Anorganik Dan Organik Sederhana Serta Persamaan Reaksinya Materi Kimia Cute766
cute766.info

Berikut Adalah Tiga Nama Senyawa Yang Mengandung Unsur Oksigen 1 Belerang Dioksida 2 Dinitrogen Brainly Co Id
brainly.co.id